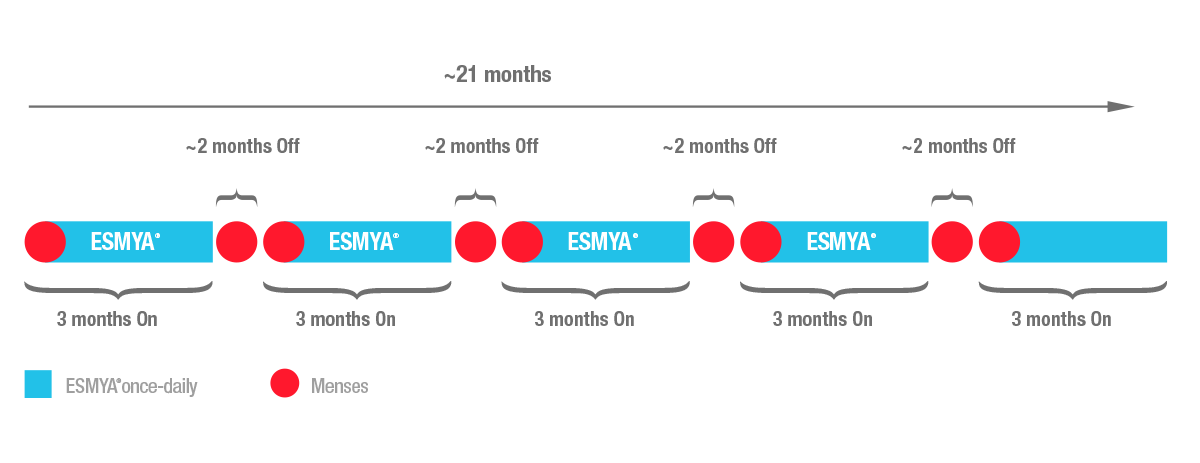
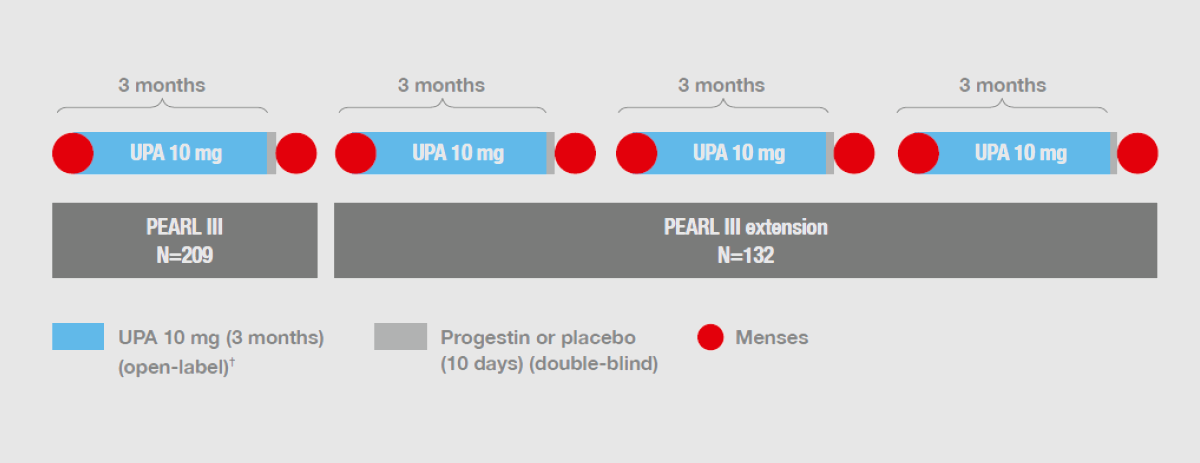
Ulipristal Acetate versus Placebo for Fibroid Treatment before Surgery43
Publications
Jacques Donnez, M.D., Ph.D., Tetyana F. Tatarchuk, M.D., Ph.D., Philippe Bouchard, M.D., Lucian Puscasiu, M.D., Ph.D., Nataliya F. Zakharenko, M.D., Ph.D., Tatiana Ivanova, M.D., Ph.D., Gyula Ugocsai, M.D., Ph.D., Michal Mara, M.D., Ph.D., Manju P. Jilla, M.B., B.S., M.D., Elke Bestel, M.D., Paul Terrill, Ph.D., Ian Osterloh, M.Sc., M.R.C.P., and Ernest Loumaye, M.D., Ph.D., for the PEARL I Study Group
N Engl J Med 2012; 366(5): 409-420
Study objectives
To demonstrate superior efficacy of Ulipristal Acetate (UPA) with concomitant iron administration versus placebo with concomitant iron administration, to reduce excessive uterine bleeding and to reduce total fibroid volume prior to surgery.
Design of PEARL I study
A Phase III, randomised, parallel-group, double-blind, placebo-controlled, multicentre study in patients with symptomatic uterine fibroids.

All patients had Hb<=10.2g/dL at screening and received concomitant administration of iron.
Efficacy
- Uterine bleeding was controlled in over 90% of women receiving Esmya® 5mg (UPA 5mg). Bleeding (PBAC score of <75) was controlled in 91% and 92% of patients in the Esmya® 5mg and UPA 10mg groups, respectively, vs. 19% in those receiving placebo, at week 13.
- More than 73% of patients, receiving UPA, reached amenorrhoea (PBAC score of ≤ 2) within 2 months. The rates of amenorrhoea were 73%, 82%, and 6% for Esmya® 5mg, UPA 10mg* and placebo, respectively.
- Amenorrhoea occurred within 10 days for 50% of patients receiving Esmya® 5mg and 70% for those receiving UPA 10mg.
- Significant reduction in fibroid volume by week 13. The median changes in total fibroid volume reduction were -21%, -12% and +3% for the 3 groups, respectively (P=0.002 for Esmya® 5mg vs. placebo, P=0.006 for UPA 10mg vs. placebo).
- Significant pain reduction, as measured by VAS scale, and improved Quality of Life.
Safety and tolerability
- The rate of the occurrence of adverse events was similar across the three groups.
- Headache and pain, discomfort or tenderness in the breasts were the most common adverse events associated with UPA, but did not occur significantly more frequently than with placebo.
- Non-physiological endometrial changes (PAEC) were observed more frequently in patients receiving ulipristal acetate than in patients receiving placebo, but these changes had resolved by the time of the follow-up assessment 6 months after the end of the treatment period.